

The weaker bonds are broken more easily than the strong. The facts and theories which are generally accepted are first reviewed, particular attention being paid both to the phenomenological aspects of surface oxide formation and decomposition, and to the occurrence of maxima in Arrhenius plots at elevated temperatures. Oxygen-containing carbons are promising supports and metal-free catalysts for many reactions. Differences in reactivity can only be attributed to differences in CH bond dissociation energies. It needs to collide with the carbonyl carbon in order to deliver its electrons to the right place. If the nucleophile hits something other than the carbonyl carbon, it will probably just bounce off. However, chemical reactions may be needed to remove other elements that might contaminate the metal.\)) and contained in cylinders packed with diatomaceous earth. Reactivity of carbon: Some current problems and trends. Crowdedness affects reactivity simply by preventing nucleophiles from easily approaching the electrophilic site in the carbonyl. It does not need to be chemically separated. Metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon, such as iron, may be extracted by reduction with carbon.Īs gold is so unreactive, it is found as the native metal and not as a compound.
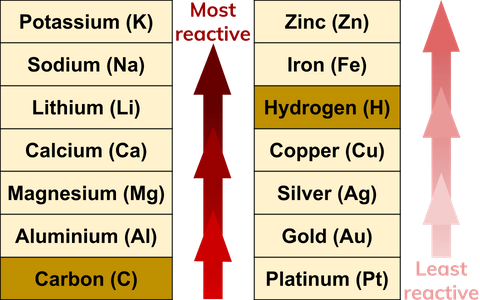
The table displays some metals in decreasing order of reactivity and the methods used to extract them. Even if the N-atom reactivity with carbon is relatively small (as suggested by our molecular beam studies of N atoms on vitreous carbon1,7).
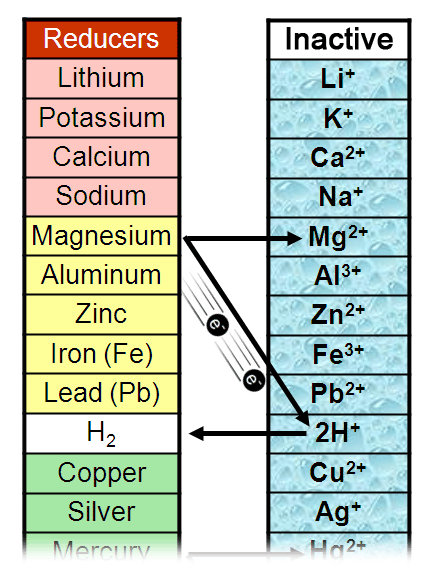
Therefore, the method of extraction of a metal from its ore depends on the metal’s position in the reactivity series. The carbon normalization index and abundance ratio established based on these molecules can indicate the reactivity and source of OC. Simultaneous optimization on bulk photogenerated-carrier separation and surface atomic arrangement of catalyst is crucial for reactivity of CO 2 photo-reduction. Rank the alkyl halides in each group in order of increasing SN1 reactivity Problems SN1.

Reduction with carbon is often used to extract these metals and requires less energy to reduce them to extract the metal. Solution: More the +I alkyl groups on electron deficient carbon. And estimates so far are too rough to gauge the fraction of the black carbon that makes. Less reactive metals, such as iron, form less stable oxides and other compounds. However, there is still a lack of direct evidence on the reactivity of black carbon in natural environments. The hard carbon puzzle: Linking material properties and electrochemical reactivity of hard carbon anodes in lithium and sodium cells.Electrolysis is commonly used to extract these metals and requires a lot of electric current (energy) to reduce them to extract the metal. Very reactive metals, such as aluminium, form stable oxides and other compounds.The method used to extract a metal from its ore depends upon the stability of its compound in the ore, which in turn depends upon the reactivity of the metal. Metals are extracted from ores, which are minerals found in the Earth’s crust that contain metal compounds.
#Reactivity of carbon series
Metal extraction and the reactivity series


 0 kommentar(er)
0 kommentar(er)
